Fossil fuels are unpopular as a source of fuel as large amounts of carbon dioxide and other greenhouse gases are produced during combustion. In order to combat global warming (and reduce the emission of greenhouse gases), it is important to look for alternative fuels.
Using Hydrogen as Fuel
Hydrogen can be burned in air. Combustion of hydrogen produces water as a by-product, which is a non-pollutant. Hence, hydrogen is classified as a clean fuel.
$$\text{H}_{2} \left( \text{g} \right) + \frac{1}{2} \text{O}_{2} \left( \text{g} \right) \rightarrow \text{H}_{2}\text{O} \left( \text{l} \right)$$
$$\Delta \text{H} =-286 \, \text{kJ}$$
Ways To Obtain Hydrogen
Hydrogen is obtained industrially from the steam reforming of methane. During this process, methane is reacted with steam at high temperature ($800-1000^{\circ}\text{C}$) and pressures (10 to 50 atm) in the presence of a nickel catalyst.
$$\text{CH}_{4} \left( \text{g} \right) + \text{H}_{2}\text{O} \left( \text{g} \right) \rightarrow \text{CO} \left( \text{g} \right) + \text{H}_{2} \left( \text{g} \right)$$
Another way to produce large quantities of hydrogen is through the cracking of hydrocarbons.
$$\text{CH}_{4} \left( \text{g} \right) \rightarrow \text{C} \left( \text{s} \right) + 2 \text{H}_{2} \left( \text{g} \right)$$
$$\text{C}_{2}\text{H}_{6} \left( \text{g} \right) \rightarrow \text{C}_{2}\text{H}_{4} \left( \text{g} \right) + \text{H}_{2} \left( \text{g} \right)$$
Hydrogen can also be obtained via the electrolysis of water, but the process is costly and produces little hydrogen. In the electrolysis of water, hydrogen ions are discharged at the cathode to produce hydrogen.
$$2H^{+} \left( \text{aq} \right) + 2\text{e}^{-} \rightarrow \text{H}_{2} \left( \text{g} \right)$$
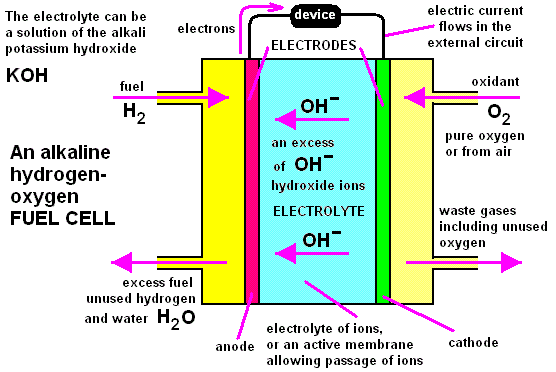
Hydrogen-oxygen Fuel Cells
A fuel cell converts chemical energy stored in chemical substances into electrical energy. However, unlike a battery, the reactants and products are not stored inside a cell. The reactants are continuously supplied from outside the cell and the products, once formed, are removed fromt he cell.
In a hydrogen-oxygen fuel cell, hydrogen is burned in oxygen to produce water. The energy is liberated as electrical energy, instead of heat energy.
Hydrogen and oxygen gas are pumped into the fuel cell at the anode and cathode respectively. Both electrodes are made of platinum.
The hydrogen molecules react with the hydroxide ions from the electrolyte (concentrated potassium hydroxide solution) to form water and electrons.
$$2 \text{H}_{2} \left( \text{g} \right) + 4 \text{OH}^{-} \left( \text{aq} \right) \rightarrow 4 \text{H}_{2}\text{O} \left( \text{l} \right) + 4\text{e}^{-}$$
The oxygen molecules react with the water molecules to form hydroxide ions.
$$\text{O}_{2} \left( \text{g} \right) + 2 \text{H}_{2}\text{O} \left( \text{l} \right) + 4\text{e}^{-} \rightarrow 4 \text{OH}^{-} \text{aq}$$
The overall reaction is the formation of water from hydrogen and oxygen.
$$2\text{H}_{2} \left( \text{g} \right) + \text{O}_{2} \left( \text{g} \right) \rightarrow 2 \text{H}_{2}\text{O} \left( \text{l} \right)$$
The advantages of hydrogen-oxygen fuel cells are:
- Fuel cells produce electricity indefinitely (when provided with a continuous supply of fuel), unlike ordinary batteries.
- Fuel cells convert chemical energy into electrical energy very efficiently so that large amounts of electricity can be produced without loss of energy.