Gases may sometimes be produced during chemical reactions. By collecting and measuring the volumes of gas produced, we can know more about the reaction which had taken place and may also use it as a reactant for another reaction.
The method used to collect a particular gas is dependent on its:
- Solubility – its ability to dissolve in water and
- Density – how “heavy” it is as compared to air.
Three common methods to collect a sample of gas are shown below:
- Displacement of Water
- Upwards Delivery
- Downwards Delivery
Displacement of Water
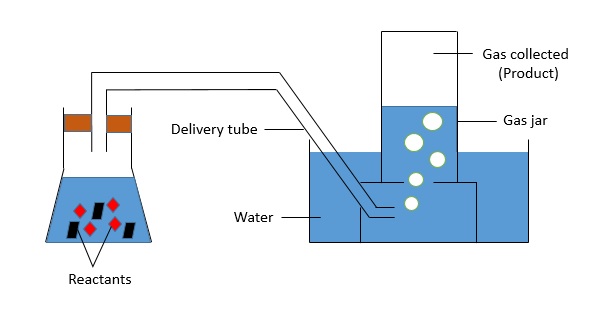
This method is suitable for collecting gases which are insoluble or only slightly soluble in water.
As the gases cannot dissolve in water and are lighter in density than water, they would rise to the top of the gas jar and be collected there. Some examples of gases collected via this way include $\text{H}_{2}$, $\text{O}_{2}$ and $\text{CO}_{2}$.
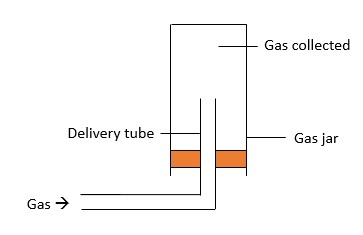
Upwards Delivery
This method is used to collect gases which are soluble in water and has a lighter density as compared to air. Some examples of gases collected this way include $\text{Cl}_{2}$, $\text{HCl}$ and $\text{SO}_{2}$. Here is a picture of the set-up:
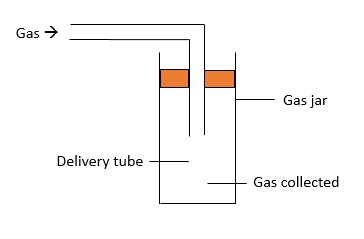
Downwards Delivery
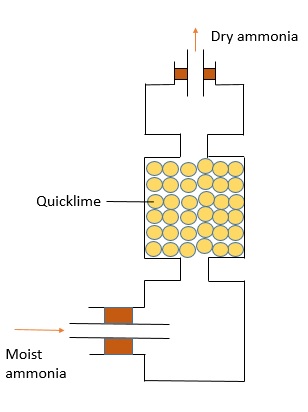
This method is used to collect gases which are soluble in water and has a heavier density as compared to air. $\text{NH}_{4}$ is one of the gases which can be collected this way.
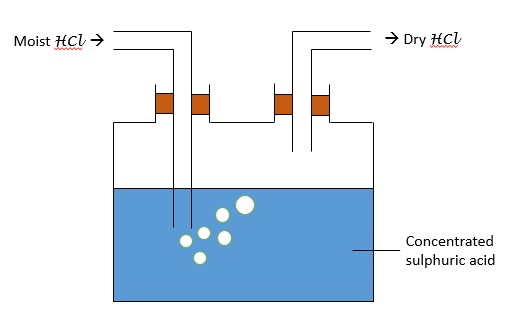
Drying of Gas Sample
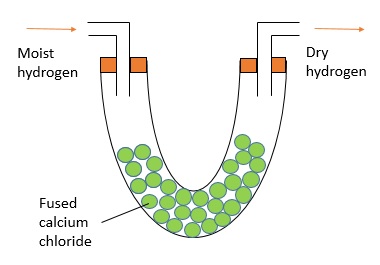
To prepare a dry sample of gas, we can pass it through drying agents like concentrated sulphuric acid, quicklime (calcium oxide) and fused calcium chloride (heated calcium chloride).
Here are the set ups for each of the drying agents:
Measurement of Volume of Gas Sample
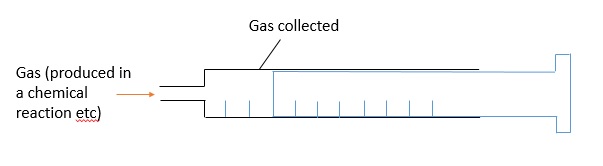
Besides collecting gases, sometimes we need to measure the volume of gas collected in order to determine the amount of reactants used, how far a reaction has progressed etc. To measure the volume of a gas, we use a gas syringe. Before the start of the experiment, the plunger is pressed fully into the barrel to expel any gas present in the syringe. As gas is produced during a reaction and enters the syringe, the plunger is pushed outwards and the volume of the gas produced can be measured:
Examples
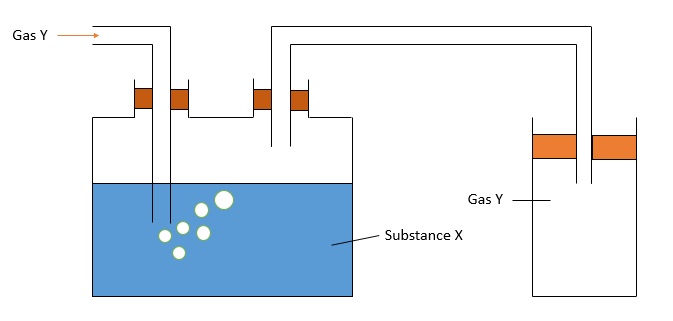
The apparatus below is used to collect a dry gas, Y. Identify Substance X and Gas Y.
A: Substance X is water; Gas Y is ammonia
B: Substance X is water; Gas Y is chlorine
C: Substance X is concentrated sulphuric acid; Gas Y is carbon dioxide
D: Substance X is concentrated sulphuric acid; Gas Y is ammonia
Click to show/hide answer
Answer is C.
Both ammonia and chlorine are soluble in water and hence, would dissolve in water if substance X is water and could not be collected. Thus, answers A and B are incorrect.
Ammonia gas would react with concentrated sulphuric acid to produce a salt and as ammonia is less dense than air, it should not be collected via a downwards delivery method. Hence, D is incorrect.
Carbon dioxide does not react with concentrated sulphuric acid and is denser than air. Therefore, C is the correct answer.
Upwards delivery method is used to collect:
A: Ammonia gas
B: Sulphur dioxide gas
C: Oxygen gas
D: Hydrogen
Click to show/hide answer
Answer is A.
Both sulphur dioxide and oxygen are denser than air and thus not suitable to be collected with upwards delivery method.
Hydrogen gas is insoluble in water and should be collected using the displacement of water method.
The examples for upwards and downwards delivery are flipped e.g Ammonia is less dense than air, not more dense
Examples are not right NH3 is less dense than air hence upward delivery should be used.Chlorine , HCl gases are more dense than air if we compare their molar masses per unit volume. Hence downward delivery should be used for these.
thank you, these really helped me understand the concepts. really appreciate it!
what is the reaction between N2 with the ethanol and we they are mixed what gas is coming out?
thank you
In examples it is written that Hydrogen gas is soluble in water but it should be insoluble.
The error has been corrected. Thank you.