Recall: A simple binary ionic compounds contains only two elements – a metal and a non-metal. When the ionic compound is in the molten state, the locked ions within the ionic structure will be free to move about (conduct electricity).
A typical setup for electrolysis of molten compounds is shown below:
 The metallic ions (cations – $M^{n+}$) will be discharged at the cathode to form a metal atom. The metallic ions are REDUCED to metal at the cathode. (because they gain electrons)
The metallic ions (cations – $M^{n+}$) will be discharged at the cathode to form a metal atom. The metallic ions are REDUCED to metal at the cathode. (because they gain electrons)
$$M^{n+} + ne^{-} \rightarrow M$$
The non-metallic ions (anions – $N^{n+}$) will be discharged at the anode to form a non-metallic atom. The non-metallic ions are OXIDISED to non-metallic atom at the anode. (because they lose electrons)
$$N^{n+} \rightarrow N + ne^{-}$$
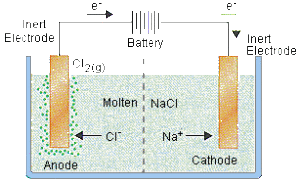
Case Study: Electrolysis of molten sodium chloride, $NaCl$
Electrodes: Carbon rods (Graphite)
Electrolytes: Molten sodium chloride
Ions present in electrolytes: Sodium ions ($Na^{+}$), Chloride ions ($Cl^{-}$)
Reaction at the CATHODE:
- $Na^{+}$ ions are attracted to the cathode.
- Each $Na^{+}$ ion gains one electron from the cathode to form one sodium atom.
- Molten sodium is formed at the anode.
- Relevant equation: $Na^{+} (l) + e^{-} \rightarrow Na(s)$ (Reduction)
Reaction at the ANODE:
- $Cl^{-}$ ions are attracted to the anode.
- Each $Cl^{-}$ ions loses one electrons to the anode to form one chlorine atom.
- Chlorine gas is liberated at anode.
- Relevant equation: $2Cl^{-} (l) \rightarrow Cl_{2}(g) + 2e^{-}$ (Oxidation)
Overall reaction:
- Every 2 moles of sodium chloride produce 2 moles of sodium metal and 1 mole of chlorine gas
- $2NaCl (l) \rightarrow 2Na(s) + Cl_{2} (g)$
Case Study: Electrolysis of molten magnesium oxide, MgO
Electrodes: Carbon rods (Graphite)
Electrolyte: Molten magnesium oxide
Ions present in electrolyte: Magnesium ions ($Mg^{2+}$), Oxygen ions ($O^{2-}$)
Reaction at the CATHODE:
- $Mg^{2+}$ ions are attracted to the cathode.
- Each $Mg^{2+}$ ion gains two electrons from the cathode to form one magnesium atom.
- $Mg^{2+} (l) + 2e^{-} \rightarrow Mg(s)$
Reaction at the ANODE:
- $O^{2-}$ ions are attracted to the anode.
- Each $O^{2-}$ ion loses two electrons to the anode to form one oxygen atom.
- $2O^{2-} (l) \rightarrow O_{2} (g) + 4e^{-}$
Overall reaction:
Every two moles of magnesium oxide produce two moles of magnesium metal and one mole of oxygen gas.- $2MgO (l) \rightarrow 2Mg(s) + O_{2} (g)$
Hello,
does metal ions (cations) always reduce in electrolysis?
Unlike electrolysis galvanic cells metals are always oxidized, is it right?