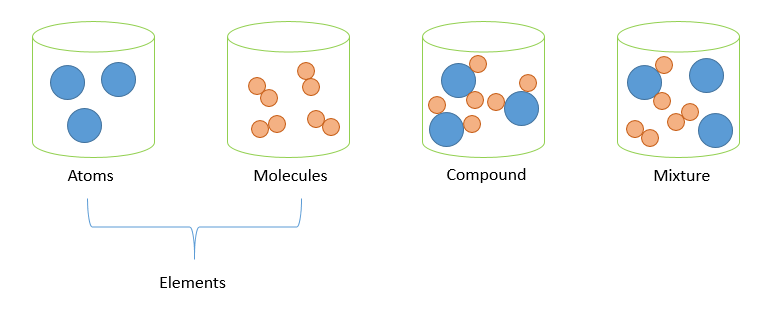
– Elements are the basic ‘building blocks” of all matter, which cannot be broken down into simpler substances by any physical and chemical methods.
– Atoms are the smallest possible unit of an element.
– Compounds are substances which consist of at least two or more elements chemically combined together.
– Molecules are the smallest possible unit of a compound and consist of at least two or more atoms chemically combined. Molecules may be comprised of atoms from the same element (eg. $Cl_{2}$) or atoms of different elements (ie. compound eg. $H_{2}O$).
– Mixtures are a combination of at least two or more atoms or molecules not chemically combined together.
 – Some differences between compounds and mixtures are shown in the table below:
– Some differences between compounds and mixtures are shown in the table below:
| Compounds | Mixtures |
|---|---|
| Pure substances, with two or more elements chemically joined together (a chemical reaction takes place when a compound is formed) | Not pure substances; where two or more elements and/or compounds are mixed together (not chemically joined together. A chemical reaction does not take place) |
| Have a fixed composition eg. water is always made up of 2 hydrogen and 1 oxygen atoms | Do not have a fixed composition |
| Cannot be separated into their components using physical methods | Can be separated into their components with physical methods like filtration, distillation |
| Have fixed melting and boiling points | Do not have fixed melting and boiling points |
| Have different chemical properties from their components | Have the properties of their components |
| Energy (may be in the form of heat or light) may be given out during their formation | Energy is usually not given out or taken in during mixing |
| Some examples include: water, carbon dioxide | Some examples include: air, salt water, soil |