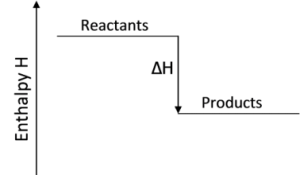A chemical reaction that involves a loss of heat energy to the surroundings, resulting in a rise in the temperature of the surroundings is called an exothermic reaction.
- Summarised version: A reaction that gives out heat to the surroundings.
- The surroundings consist of the reaction container, the laboratory room and anything else other than the reactants and products.
As the exothermic reaction progresses, the temperature of the surroundings increases.
- The reaction container becomes hot.
The total energy content of the products of an exothermic reaction is lower than the total energy content of the reactants. This information can be displayed in the form of a graph, as shown below.
$\Delta \text{H}$ stands for the change in enthalpy.
- $\Delta \text{H}$ represents the heat of reaction (enthalpy change).
- $\Delta \text{H}$ is equal to the difference between the energy content of the products and the reactants.
- As heat energy is given out in an exothermic reaction, the $\Delta \text{H}$ for an exothermic reaction is negative.
Examples of Exothermic Reaction
Combustion of carbon is an exothermic reaction:
- $\text{C}\left( \text{s} \right) + \text{O}_{2}\left( \text{g} \right) \rightarrow \text{CO}_{2}\left( \text{g} \right)$, wherby $\Delta \text{H} =-400 \text{ kJ mol}^{-1}$
- The complete reaction (combustion) of 1 mole of C with 1 mole of $\text{O}_{2}$ to form 1 mole of $\text{CO}_{2}$ gives out a total of 400 kJ of heat energy.
- 1 mole of $\text{C}$ and 1 mole of $\text{O}_{2}$ have a higher energy content than 1 mole of $\text{CO}_{2}$.
Combustion of methane is another exothermic reaction:
- $\text{CH}_{4} + 2\text{O}_{2} \rightarrow \text{CO}_{2} + 2\text{H}_{2}\text{O}$, whereby $\Delta \text{H} =-882 \text{ kJ mol}^{-1}$