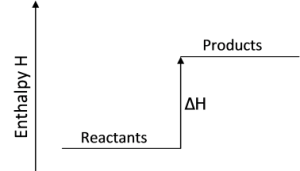A chemical reaction that involves a gain of heat energy from the surroundings, resulting in a drop in the temperature of the surroundings is called an endothermic reaction.
- Summarised version: A reaction that takes in heat from the surroundings.
- The temperature of the surroundings decreases. (The reaction container becomes colder.)
The energy content of the products is higher than the energy content of the reactants.
As heat energy is gained in an endothermic reaction, the $\Delta \text{H}$ is positive.
Examples of Endothermic Reactions
Formation of carbon disulphide is an endothermic reaction:
- $\text{C}\left( \text{s} \right) + 2 \text{S} \left( \text{s} \right) \rightarrow \text{CS}_{2} \left( \text{s} \right)$, whereby $\Delta \text{H} =+100 \text{ kJ mol}^{-1}$.
- This means that 100 kJ is required to form 1 mole of carbon disulphide from 1 mole of carbon and 2 moles of sulphur.
- 1 mole of carbon disulphide has a higher energy content than 1 mole of carbon and 2 moles of sulphur.
Decomposition of calcium carbonate:
- $\text{CaCO}_{3} \rightarrow \text{CaO} + \text{CO}_{2}$, whereby $\Delta \text{H} =+384 \text{ kJ mol}^{-1}$.