From collision theory for rate of chemical reactions, we know that there are a few factors which affect the rate of a reaction:
- Concentration
- Pressure
- Particle size
- Temperature
- Presence of catalyst
Effect Of Concentration On Rate Of Chemical Reactions
An increase in the concentration of one or more of the reactants will increase the rate of reaction.
Why?
When the concentration of one or more of the reactants increases, the following sequence of events may occur:
- There will be more reactant particles in a given volume (i.e. high number of reactant particles per unit volume).
- Reactant particles will collide more often.
- Number of collisions per unit volume will increase.
- Number of effective collisions increases.
- Rate of reaction increases.
Effect Of Pressure On Rate Of Chemical Reactions
A change in the pressure will only affect the rate of reaction for chemical reactions involving gaseous reactants. An increase in the pressure will lead to an increase in the rate of reaction.
Why?
When the pressure increases, the following sequence of events may occur:
- The increase in pressure forces the gaseous reactant particles closer together.
- Number of reactant particles per unit volume increases.
- Number of collisions per unit volume increases.
- Number of effective collisions increases.
- Rate of reaction increases.
High pressure is used frequently in industrial processes to improve the rate of chemical reactions. This is because a higher rate of reaction will mean more products are made per unit time (i.e. more profits for the companies). A common example of such an industrial process is the Haber Process, whereby a pressure of 200 atm is used to speed up the process and increase yield.
Effect Of Particle Size On Rate Of Chemical Reactions
A decrease in the particle size of a solid reactant will increase the rate of reaction.
Why?
When the particle size of a solid reactant is decreased, the following sequence of events may occur:
- The particle size of a solid reactant is decreased by breaking up the solid reactant into smaller pieces.
- This action will increase the total surface area.
- The area of contact between the reactant particles increased.
- Number of collisions per unit time increases.
- Number of effective collisions per unit time increases.
- Rate of reaction increases.
Effect Of Temperature On Rate Of Chemical Reactions
An increase in the temperature will increase the rate of reaction for most chemical reactions.
Why?
When the temperature is increased, the following sequence of events may occur:
- Reactant particles have more kinetic energy (i.e. they move faster).
- Frequency of collision between reactant particles increases AND a larger number of reactant particles have energy equal to or more than the activation energy.
- Number of collisions per unit time increases.
- Number of effective collisions per unit time increases.
- Rate of reaction increases.
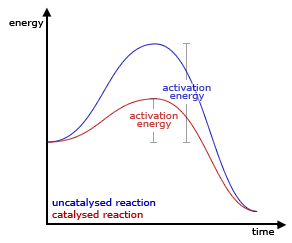
Effect Of Catalyst On Rate Of Chemical Reactions
A catalyst is a chemical substance that changes the rate of reaction without itself undergoing any permanent chemical change at the end of the reaction.
A catalyst work by providing an alternative reaction pathway for the reaction, i.e. one that has a much lower activation energy. (As shown in the figure above)
This means that: The presence of a catalyst will increase the rate of reaction.
Why?
With a catalyst, the following sequence of events may occur:
- An alternative reaction pathway with a lower activation energy is now available.
- More reactant particles will have sufficient energy to overcome the energy barriere.
- Number of effective collisions per unit time increases.
- Rate of reaction increases.
Notes about catalysts:
- Even though a catalyst remains chemically unchanged at the end of a reaction, its physical features such as colour and texture may be changed. (i.e. May change from a solid block to a powdered form)
- A catalyst is generally specific in action, i.e. can only catalyst one type of reaction. Different reactions will need different types of catalysts.
- A catalyst does not affect the types or quantity of products formed in a chemical reaction. It only allows the products to be obtained in a shorter amount of time.
- Transition metals and their compounds make good catalysts.
- Enzymes are biological catalysts.