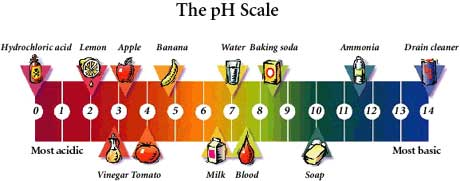
The pH scale (as shown in the figure above) is used to measure the acidity or alkalinity of an aqueous solution. The pH scale is numbered between 0 to 14.
For reference, the equation for pH is given by:
$$\text{pH} =-\text{lg} [\text{H}^{+}]$$
pH is given by the negative logarithm of the concentration of hydrogen ions ($[\text{H}^{+}]$). You can see that pH depends on the concentration of $\text{H}^{+}$ only and not on the strength of the acid.
A neutral solution has a pH value of 7. (E.g. pH of pure distilled water) This means that the concentration of $\text{H}^{+}$ ions is equal to the concentration of $\text{OH}^{-}$ ions.
An acidic solution has a pH value of less than 7. (E.g. pH of sulphuric acid)
- This means that the concentration of $\text{H}^{+}$ ions is more than the concentration of $\text{OH}^{-}$ ions.
- The smaller the pH value, the more acidic the solution is.
- A solution with a pH value of 2 is more acidic than a solution of pH value of 3.
An alkaline solution has a pH value of more than 7. (E.g. pH of aqueous sodium hydroxide)
- This means that the concentration of $\text{H}^{+}$ ions is less than the concentration of $\text{OH}^{-}$ ions.
- The bigger the pH value, the more alkaline the solution is.
- A solution with a pH value of 10 is more alkaline than a solution of pH value of 9.
Indicators
Indicators are organic substances that have different colours in acidic and in alkaline solutions.
- Each indicator has an acidic colour, an alkaline colour and a pH value at which it changes colour.
A table of common indicators are shown below.
| Indicator | Colour in strongly acidic solution | pH at which colour change | Colour in strongly alkaline solution |
|---|---|---|---|
| Methyl orange | Red | 4 | Yellow |
| Litmus paper | Red | 7 | Blue |
| Phenolphthalein | Colourless | 9 | Red |
| Screened methyl orange | Red | 4 | Green |
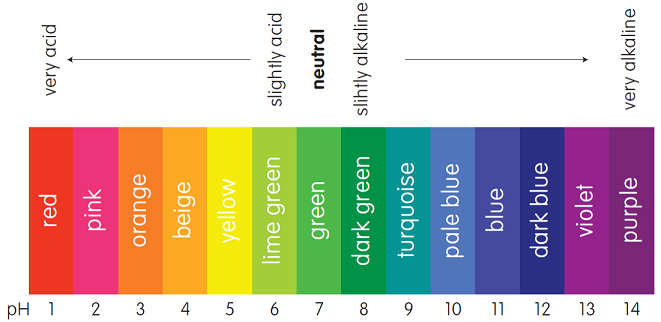
A Universal Indicator (or pH Indicator) gives different colours at different pH numbers. The colours at the various pH numbers are shown in the figure below:
pH Value & Agriculture
Most plants grow best when the pH value of the soil is about 6.5.
- Plants do not grow well in soil that is too acidic or too alkaline.
- Most types of soil are slightly acidic or alkaline with pH values between 6 to 8.
Soil can become too acidic from excessive use of chemical fertilizers and from acid rain.
Excess acid in soil can be neutralized by adding lime (calcium hydroxide).