The basic building blocks of matter are atoms – small individual particles.
Atoms are made up of three basic particles:
- proton
- neutron
- electron
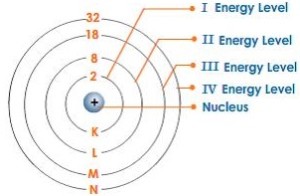
Proton and neutron are found in the nucleus of the atom. The nucleus is located at the centre (core) of the atom. Proton and neutron are also known as nucleons.
Electrons can be thought to be “orbiting” the nucleus at various energy levels (or known as electron shells).
Properties that you must know for p, n and e:
| Proton (p) | Neutron (n) | Electron (e) | |
|---|---|---|---|
| Relative Mass | 1 | 1 | $\frac{1}{1840}$ |
| Relative Charge | +1 | 0 (Neutral) | -1 |
What does the numbers on relative mass means?
- Protons and neutrons are almost the same weight (only some minute weight difference between the two)
- Electrons is more than 1800 times lighter than proton/neutrons
Some terminology that you need to know:
Proton number is the number of protons in an atom- Nucleon number is the total number of nucleons (protons and neutrons) in the nucleus
An atom is electrically neutral. $\rightarrow$ The number of protons and electrons are the same.
Number of neutrons = Nucleon number – proton number
In chemistry, we denote substances with their symbols accompanied by various information about the number of protons, electrons and neutrons. For instance, $^{a}_{b}X$ means that the atom X has:
- number of nucleons: a
- number of protons: b
- number of electrons: b
- number of neutrons: a – b