Macromolecules are extremely large molecules which contains thousands of atoms in each molecules. Examples of macromolecule: Diamond, Graphite, Silica, Poly(ethene)
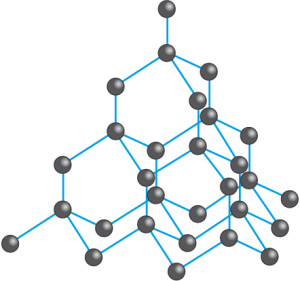
Diamond
- Diamond is made up of carbon atoms arranged in a tetrahedral structure as shown above.
- Notice that each carbon atom is bonded to four other carbon atoms. This is because carbon has four valence electrons to form four covalent bonds to 4 other other carbon atoms.
Properties of diamond:
- Hard
- Diamond is the hardest substance known.
- The carbon atoms in diamond are not able to slide over one another because all of the carbon atoms are bonded together by very strong covalent bonds to form a giant molecular structure. The strong covalent bonds holding the carbon atoms together and the rigid tetrahedral structure gives diamond its hardness.
- High melting and boiling point
- A lot of energy is needed to break the numerous strong covalent bonds between the carbon atoms
- Does not conduct electricity
- Each carbon atoms uses all its 4 valence electrons for bonding. Hence, it has no free mobile electrons to conduct electricity.
Applications of diamond:
Due to its hardness, diamond is suitable to be used for cutting other hard substances.
- Diamonds can be embedded into drill bits and saws.
- Diamond is used to cut glass and metals.
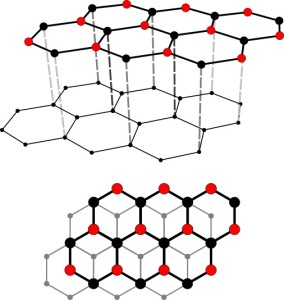
Graphite
- Graphite has a layered structure – layers of carbon atoms.
- In each layer, all the carbon atoms are bonded together by strong covalent bonds in a 2-dimensional lattice. Each carbon atom is bonded to three other carbon atoms in a hexagonal arrangement. This means that each carbon atom only uses three valence electrons to form three covalent bonds with three other carbon atoms. Note that this means that there will be one free electron.
- The layers of carbon atoms are held together bu weak Van der Waal’s forces of attraction.
Properties of graphite:
- Soft
- The weak Van der Waal’s forces of attraction between the layers are easily overcome and this enables the layers to slide over one another.
- High melting and boiling point
- A lot of energy is required to break the numerous strong covalent bonds between the carbon atoms
- Conducts electricity
- Remember that there is one free electron available to each carbon atom. The spare electrons are free to move (‘delocalised’), so they can flow along the layers. Hence, grahite is able to conduct electricity
Applications of graphite:
- Graphite is soft and slippery. This makes it suitable for use as a lubricant, especially for hot machines. This is because it does not decompose at high temperature. (unlike oil)
- It is also used in pencil leads as the layers of atoms slide off the pencil onto the paper
- Graphite is also used for contacts in electrical motors.
- Graphite is unreactive and can withstand high temperature, so it is used as electrodes in the extraction of metals by electrolysis.
Silicon
- Silicon has a similar structure as diamond.
- All the silicon atoms are bonded together by strong covalent bonds in a tetrahedral arrangement.
- Each silicon atom has four valence electrons which are used to form four covalent bonds to four other silicon atoms.
- This will result in silicon having similar properties as diamond.
- Silicon is hard though not as hard as diamond.
- Pure silicon does not conduct electricity, but becomes a semi-conductor when impurities are added to it.
Silica (Silicon Oxide)
- Silicon oxide has a similar structure as silicon and diamond
- Each silicon atom is covalently bonded to four other oxygen atom in a tetrahedral arrangement.
- This will result in silicon oxide having similar properties as diamond and silicon.
- Silica is hard though not as hard as diamond.